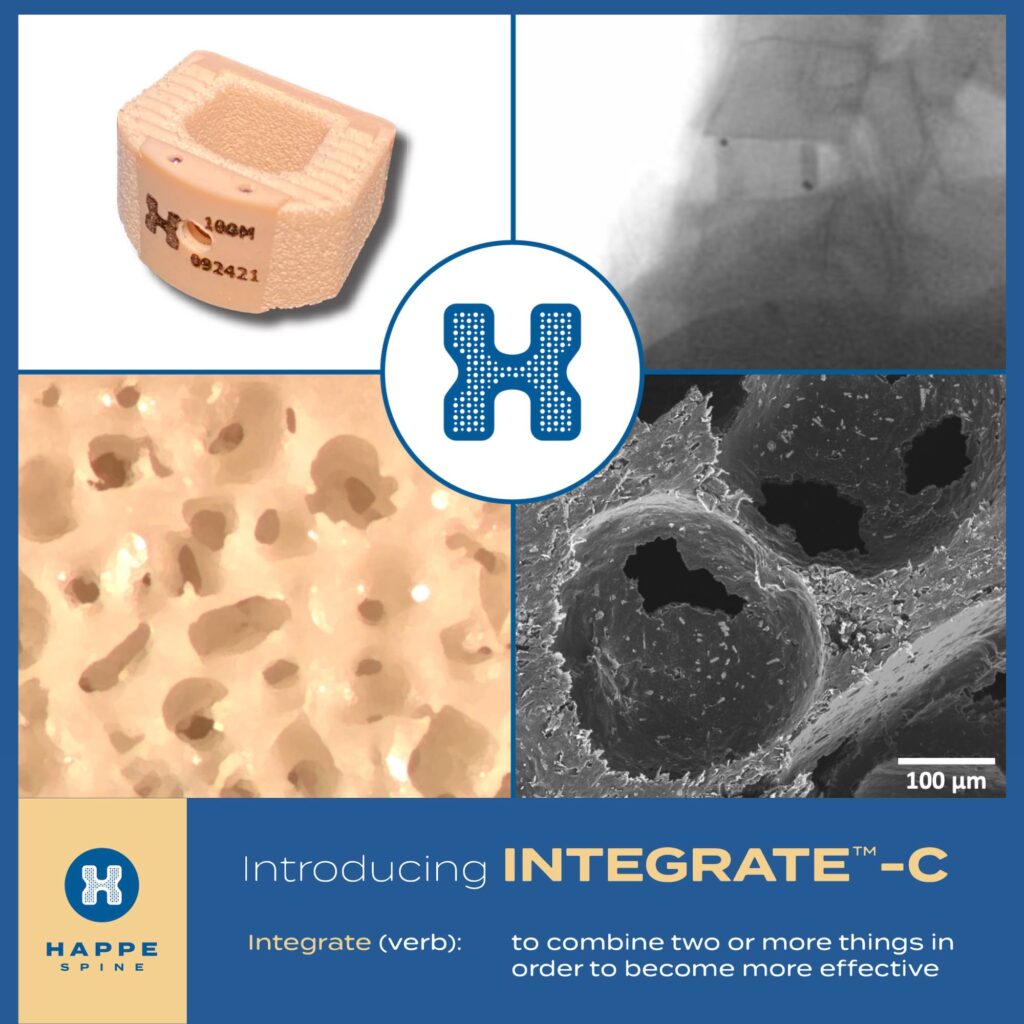
Grand Rapids, Mich., May 8, 2023 — HAPPE Spine, a medical device company focused on bringing innovative materials to orthopaedic implants, announces that the company’s INTEGRATE-C™ Interbody Fusion System has received 510k clearance from the FDA.
Powered by the HAPPE® platform, INTEGRATE-C™ is the first interbody fusion cage that is fully integrated with porosity and hydroxyapatite to provide a superior healing environment. The interconnected, cancellous porosity promotes bone ingrowth from endplate-to-endplate. Hydroxyapatite is exposed on all surfaces to promote cell signaling and bone on-growth. The INTEGRATE-C™ is both radiolucent and radiovisible for superior intra-operative and post-operative imaging.