Holland, Mich. – October 29, 2020 – Genesis Innovation Group’s cultivate(MD) Seed Fund, a fund focused on investments into early-stage healthcare companies with innovative technologies, announces that one of their portfolio companies, Norfolk, VA based Embody, Inc. has received FDA 510(k) clearance for their TAPESTRY® Biointegrative Implant for Tendon and Ligament Repair.
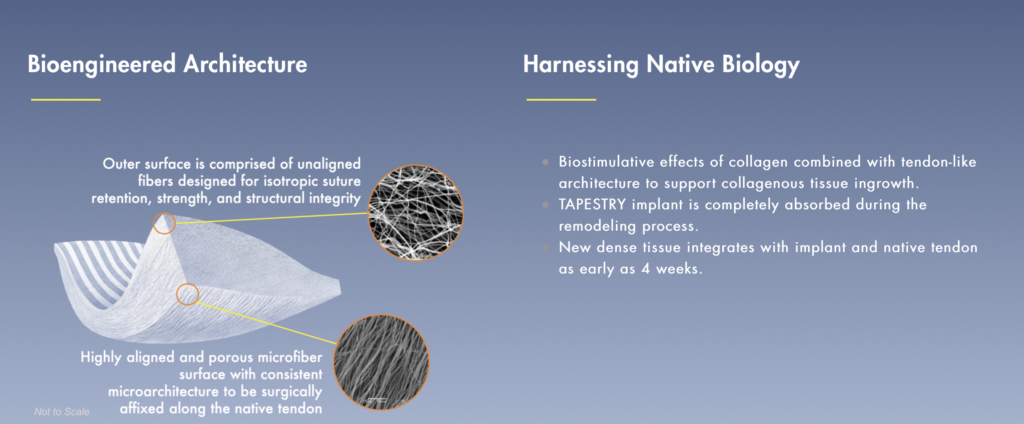
Embody is a privately-held medical device company developing novel collagen-based technologies for sports medicine and soft tissue repair. TAPESTRY is a bioengineered collagen-based implant with a proprietary micro-architecture specifically designed for tendon and ligament repair.
With initial funding through the Defense Advanced Research Projects Agency (DARPA) Atoms to Products Program and follow-on investment from cultivate(MD) Capital Seed Fund and others, Embody has pioneered additive manufacturing and advanced biofabrication techniques to assemble collagen from the molecular scale to produce bioengineered medical devices specifically designed for soft tissue repairs, a market estimated at 2.4 million annual procedures in the U.S.
“This FDA clearance represents a major milestone in the advancement of Embody’s mission to improve outcomes for patients who suffer from tendon and ligament injuries. TAPESTRY represents a significant advancement in collagen science and provides surgeons with a compelling solution with broad clinical utility for tendon repair procedures,” said Jeff Conroy, CEO of Embody. Kevin F. Bonner, MD, an orthopedic surgeon and Director of the Research Foundation at The Jordan-Young Institute in Virginia Beach, VA stated, “There is clearly a clinical need to further improve upon our current healing rates in the treatment of many tendon and ligament injuries. Embody’s TAPESTRY implant represents a breakthrough in implant design that combines the benefits of collagen with the structural integrity required for demanding tendon and ligament applications from foot & ankle to complex shoulder repair procedures.”
“We are really excited about our relationship and our partnership with Embody. This FDA clearance with Tapestry opens up an opportunity to help address real unmet needs for patients” said Rob Ball, Chairman of Genesis Innovation Group and Director, cultivate(MD) Seed Fund.
Embody is prepared to proceed with the commercialization of Tapestry in the U.S. immediately.
About cultivate(MD) Capital Funds
As a medical device venture capital fund, cultivate(MD) is dedicated to bringing emerging health care innovations to market, with a special focus on medical device and orthopedic technologies. cultivate(MD) is focused on investing in early-stage healthcare companies with innovative technologies that have demonstrated evidence of effectiveness.
This press release does not constitute an offer to sell or solicitation of an offer to buy any securities in any offering of securities. There will not be any sale of any securities in any state or jurisdiction in which such offering, sale or solicitation would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction.
About Embody
Embody is pioneering the next generation regenerative platform for the repair of tendon and ligament injuries with novel collagen-based bio-fabrication techniques and products for the fast-growing sports medicine market. Founded in 2014 and funded with $20 million in DARPA & DOD funding, the company is developing unique biomaterials with an initial focus on orthopedic applications including Achilles, rotator cuff and knee ligament repair.
Contact:
Jill Wachter
jill.wachter@genesisinnovationgroup.com

